As the largest organ of the human body, the skin literally provides a major barrier exposed to environmental stimuli and pathogens. Painful inflammation can be mounted if this barrier is compromised – as anyone who has ever had a sunburn knows. But exactly how this is triggered was not understood in detail until now. "In our study, we took a closer look at the processes involved," explains Prof. Dr. Florian Schmidt, who heads a research group at the Institute of Innate Immunity at the University Hospital Bonn.
UV stress triggers signal chain
UV light is very high in energy. When it hits the skin, it can therefore damage important cellular molecules, sparking inflammation as a common consequence. However, it was unclear how this exactly happens. "We have now been able to show that a known cellular stress signaling pathway can trigger these inflammatory responses," explains Schmidt, who is also a member of the Transdisciplinary Research Area (TRA) "Life and Health" and the ImmunoSensation2 Cluster of Excellence at the University of Bonn.
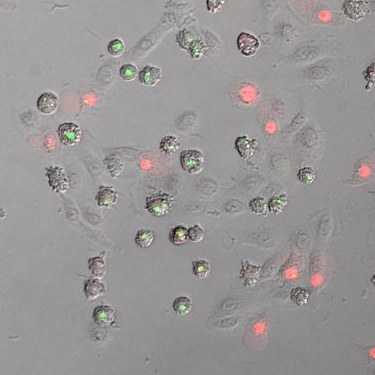
The cell's own "engineering offices," the ribosomes, normally assemble proteins based on the instructions in the genetic material. When this is impaired due to UV damage, they sound the alarm: They trigger the so-called ribotoxic stress response. It has been known for years that this causes a signaling cascade resulting in the activation of an enzyme called p38. "Our research shows that p38 molecularly modifies NLRP1, a critical switch for inflammation in the skin, and thus activates it in a novel way. This initiates the assembly of inflammasomes from many molecular building blocks."
Inflammasomes are powerful weapons of the innate immune system. Among other things, these complex molecular machines can convert inactive messenger substances for inflammation into their active form. At the same time, they ensure that numerous holes are formed in the cell membrane. This allows messenger substances to reach the outside and thus call the body's own defense forces to its aid. Ultimately, the holes lead to the death of the cell: At some point, it practically explodes and empties its contents into the tissue. The molecules that are now abruptly released from within the cell are another warning sign for the immune system.
Viruses also activate p38
Interestingly, p38 is not only activated by excessive sunbathing. "We were able to show that mosquito-borne viruses can activate NLRP1 through p38 as well," emphasizes Lea-Marie Jenster, a PhD student in the Schmidt lab and the lead author of this study. "These include, for instance, chikungunya virus, which is a major problem in parts of Africa and Asia and could also reach Germany in the wake of climate change." Viruses probably even trigger the activation of p38 via several different pathways.
"P38 is a molecular information hub in the skin, in which various warning signals converge - similar to the fire department's control center," Schmidt explains. "However, not every incoming call for help immediately triggers the assembly of inflammasomes - this only happens when the number and intensity of alerts exceed a certain threshold." This regulation is important, since inflammasomes are dangerous weapons which cause considerable collateral damage. For example, the strong inflammation that is triggered causes parts of the skin tissue to perish.
Sometimes, however, inflammasomes are controlled not strictly enough - as in the case of sunburn or even some autoimmune diseases. Perhaps p38 opens up a new possibility to specifically suppress such exuberant immune reactions in the skin.
Participating institutions:
In addition to the University Hospital of Bonn and the University of Bonn, the University of Melbourne (Australia) and Boston Children's Hospital (USA) were involved in the study.