Getting started

Information AAV
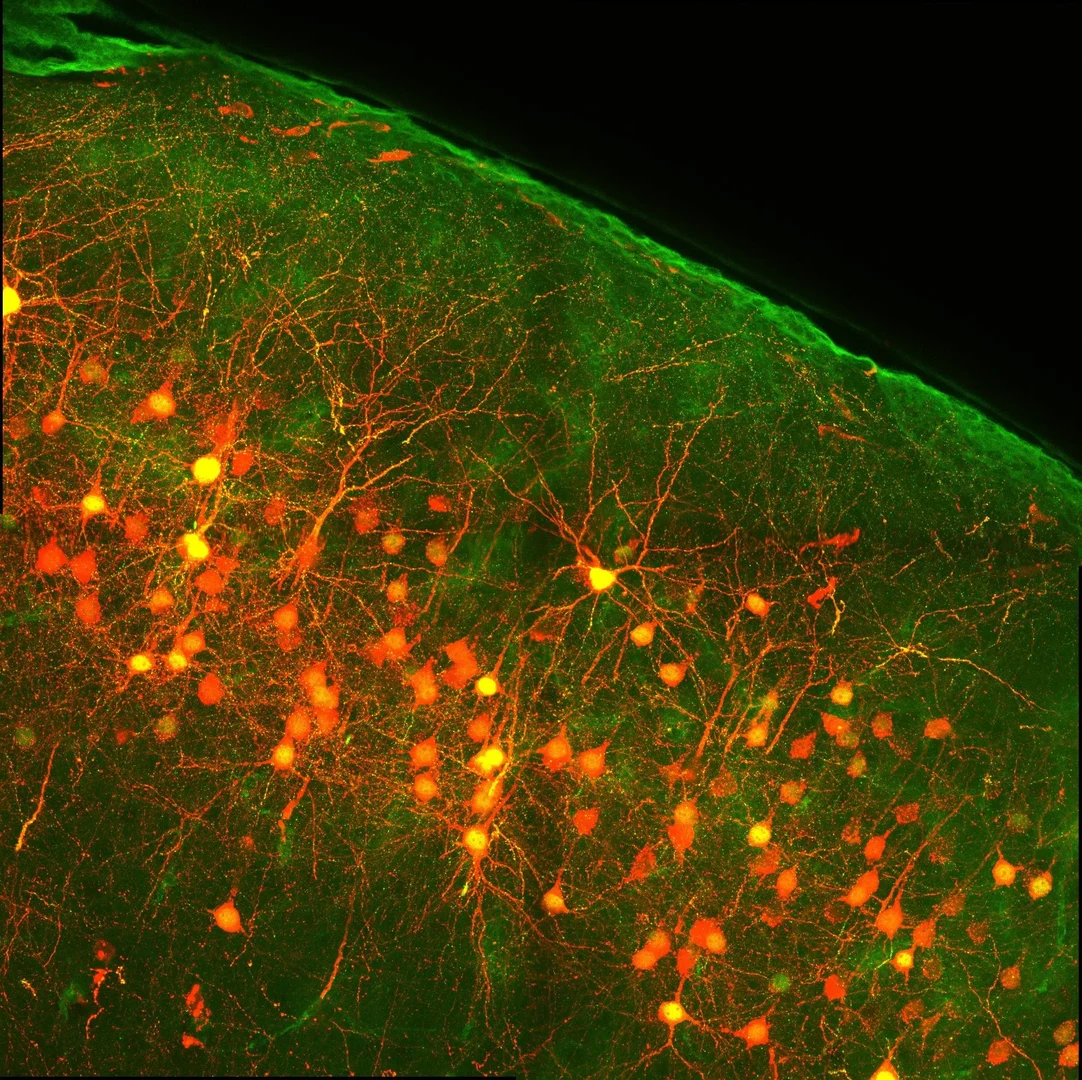
Adeno-associated viruses in research
A significant advantage of viruses is their capacity to penetrate cellular membranes and deliver DNA into the cell. This property is exploited in a research context to deliver transgenes into target cells both in vitro and in vivo. The Core Facility produces adeno-associated viruses for research purposes only.

AAV expression plasmid
In recombinant AAVs, the viral genes are substituted by an expression cassette consisting of a promoter, the protein of interest, and regulatory elements. Two inverted terminal repeats flank this cassette that can comprise up to 4.7 kbp.

Virus production and purification
In the Core Facility, we produce recombinant AAVs using the adenovirus-free helper production method. The AAV expression plasmid, the Rep/Cap encoding plasmid that defines the serotype of the AAV, and a plasmid encoding the necessary adenoviral helper genes are co-transfected to generate AAVs.

Virus serotypes
AAVs have several advantages, including the availability of different serotypes. These serotypes differ in their cellular tropism, allowing for targeted delivery to specific cell types and organs. The choice of purification method—either heparin affinity chromatography or iodixanol density gradient ultracentrifugation—depends on the particular serotype produced.
Workflow
Step 1
Contact Us
Reach out to us via email to discuss your project goals and specific requirements.
Step 2
Submit Request Form
Fill out our request form to initiate the project.
Step 3
Provide DNA
Drop off your DNA material, and we will start the plasmid cloning or virus production process.
Step 4
Pick up Virus
Once production is complete, you are welcome to pick up your DNA or virus.
Larissa Kündgen
Department for Epileptology, building 83
Venusberg-Campus 1
53127 Bonn
Dr. Anne Quatraccioni
Department for Epileptology, building 83
Venusberg-Campus 1
53127 Bonn
Prof. Dr. Susanne Schoch McGovern
Department for Epileptology, building 83
Venusberg-Campus 1
53127 Bonn
Address
How to find us
Arrival by bus and campus maps
From Bonn main station you can take the bus line 600 (direction Ippendorf Altenheim) and 601 (direction Venusberg) to the bus stop “Uniklinikum Süd”.
Further information including detailed maps can be found here.
The Core Facilities thank the German Research Foundation for continuous support.
